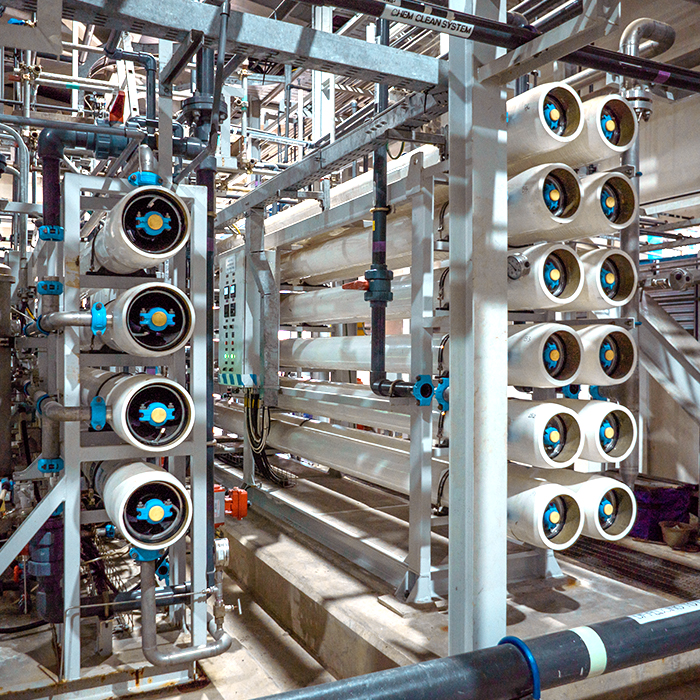
The reduction of total dissolved solids (TDS) can be achieved by Ion Exchange or Membrane processes. We have proposed a membrane-based system, more specifically an RO system, because of its compatibility, durability, robustness for different water qualities, and environmental friendliness.
Osmosis is a natural phenomenon that occurs when two solutions of different concentrations are separated by a semi-permeable membrane. Under this condition, a pressure is created, which forces the solvent through the membrane from the dilute solution to the concentrated solution. This movement of pure solvent is called osmosis, and the pressure at which it occurs is called osmotic pressure.
When external pressure is gradually applied to the concentrated solution, a stage is reached where there is no flow of pure solvent through the membrane. If excess pressure is applied beyond this point, pure solvent begins to flow through the membrane from the concentrated solution to the dilute solution. This process is called reverse osmosis (RO).
RO technology uses a high-pressure pump to force a portion of the feed water through a semi-permeable membrane. The amount of permeate (product water) produced varies directly with the feed water pressure and temperature. Since the bulk of the contaminants remain on the feed water side of the membrane, over time they can foul or scale the membrane. To prevent this, a portion of the feed water is directed to drain or other uses. This stream is called the concentrate, and the amount required depends on the quantity and type of contaminants in the water.